Certified Quality Assurance
Throughout the company's history, TransPerfect has shown an unmatched commitment to quality. As the first major language services provider to be certified to the ISO 9001 and ISO 17100 standards, TransPerfect is the undisputed industry pioneer in quality assurance. Additionally, TransPerfect also achieved ISO 18587 certification for its AI-Enabled translation services.
Environment Management System
TransPerfect Medical Device Solutions Certifications
Our Medical Device Solutions division holds specialty certifications to ISO 13485 and ISO 14971 in addition to ISO 9001, ISO 17100 and ISO 18587, and holds the only patent in translation risk management.
Occupational Health and Safety Management System
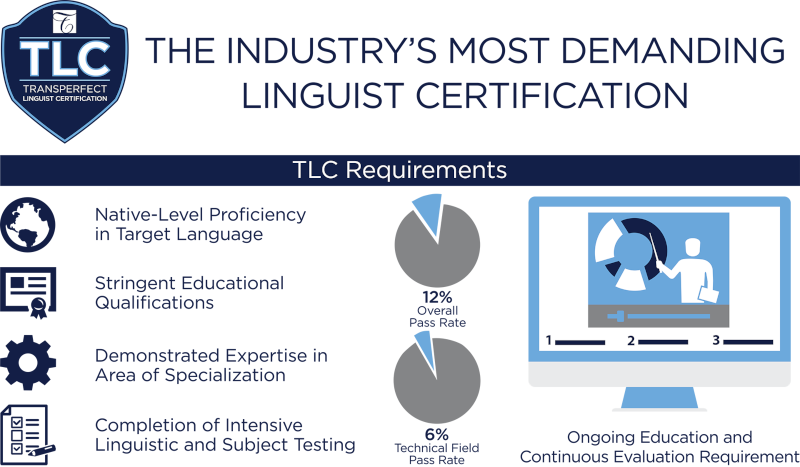
TransPerfect Linguist Certification Program
While various professional groups and organizations have historically provided credentials for professional linguists, none of these addressed critical elements of linguist performance like subject-matter expertise and overall competency — so TransPerfect introduced the TransPerfect Linguist Certification (TLC) program. The TLC program was the first comprehensive system to assess linguist skill in the areas that matter most to clients.